Overview
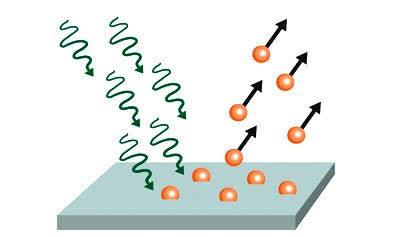 Diagram
showing the basics of photoelectric effect
Diagram
showing the basics of photoelectric effect
History
 German
Physicist Heinrich Hetz
German
Physicist Heinrich Hetz
He noticed carefully that increasing the brightness of the light caused
increased production of electrons even though the energy of the electrons did
not increase full stop on the other hand increasing the frequency of the light
cause the emission of electrons of increased energy even though the number of
electrons emitted did not increase. This phenomena could not be explained by
henrich but later by a young scientist name Albert Einstein in 1905.
Albert Einstein was later awarded the Nobel prize for his achievement in
explaining the photoelectric effect completely.
Explanation
Before you understand the photoelectric effect we shall understand a few
important terms at first:
1. Planck's constant => a fundamental physical constant denoted by letter
'h'. The frequency of a photon when multiplied by planck's constant gives us
the energy of that Photon. So,
Let, f
requency of photon = v
Plank's constant = h
Total energy of Photon (E) = vh
Where,
Plank's constant (h) = 6.62607015 × 10-34J/s
2. Threshold frequency => The minimum frequency that a photon must have to
cause emission of electrons from metal surface viz. well cause photoelectric
effect. It is different for different metals because of their work functions.
Remember that photoelectric effect is also possible for non-metals to a
limited extent due to their high threshold frequency and sometimes although
rarely with fluids such as air and water.
3. Work function => The minimum amount of energy required to move an
electron to a infinite seperation from the surface of a solid usually a metal
is called the work function of that solid.
More is the work function, more will be the threshold frequency, infact for
photoelectric effect to occur the threshold frequency must always be equal to
the work function of the solid of not more.
So, here's what the photoelectric effect is all about -
We all know that light consists of photons. Suppose a break off light falls on
a metal surface such that,
Frequency of photon = v
Plank's constant = h
Work function of metal = W
Threshold frequency = f
For photoelectric effect to actually happen,
'v' must always be greater than 'f' i.e. v > f
Then the prom will be able to knock off an electron from the metal surface
given,
v > W
So, to take the electron or of the metal surface 'W' amount of energy is used
up and the electron so emitted is now left with (vh - W) amount of energy.
This electron is not free and can constitute current if allowed to flow by use
of conductors such as wire. This current can be harvested as electricity.
Factors affecting photoelectric effect
The photocurrent i.e. the current produced from the emission of electrons due
to photoelectric effect depends mainly on for factors :-
1) Frequency of Incident radiation
Increase in the frequency of incident light causes emission of electrons of
more energy although there is no noticeable increase in the number of
electrons emitted.
2) Intensity of Incident radiation
Increase in the intensity of incoming radiation causes increase in the number
of electrons emitted although there is rise in the energy levels of the
electrons emitted.
3) Potential difference across electrodes
With an increase in the potential difference between the electrodes, the net
flow of photocurrent increases.
4) The material used
Metals are preferable because they can loose electrons more easily, although
glass is also sometimes used.
So, to increase the overall efficiency of a photoelectric system increasing in
the frequency and intensity of the radiation as well as the potential
difference across the electrodes and a good metal used like copper will yield
the best result.
Comments
Post a Comment